4. Alanine is the second simplest amino acid (behind glycine), but there is a lot to it. Alanine scanning involves mutating certain amino acids in a protein to alanine. Why is this process very valuable to biochemists?
From Quiz Amino Acids - Why They Matter
Answer:
It allows protein structure and function to be probed
Alanine is chemically simple and so by replacing other amino acids of a protein with alanine, it may be deduced what role these replaced residues play (either functionally or structurally). For example, glutamate, which usually exists as a negatively charged form, may be involved in a charge-charge interaction with a positively charged residue (arginine, for example). If there is indeed a charge-charge interaction, and if this interaction has any significance in either the structure or the function of the protein, this will be apparent following alanine mutagenesis, since alanine is incapable of mimicking the negative charge of glutamate.
Alanine is also one of the most important amino acids in gluconeogenesis - the process of synthesising glucose from non-carbohydrate sources in times of starvation/intense exercise. In active muscles, pyruvate is released when glucose is metabolised. This pyruvate can be converted back to glucose in the liver, but is unable to travel in the blood. Pyruvate is therefore either converted in the muscle into lactate or alanine, which are then transported to the liver via the blood, where they are converted to glucose. When carried as lactate, this is known as the Cori cycle, and when carried as alanine, it is the alanine cycle.
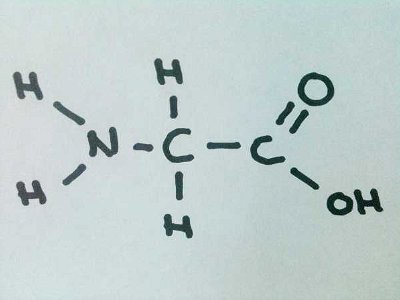 As well as giving me a chance to display my artistic skills, this quiz tests your knowledge of amino acids based primarily on their structures.
As well as giving me a chance to display my artistic skills, this quiz tests your knowledge of amino acids based primarily on their structures.  As well as giving me a chance to display my artistic skills, this quiz tests your knowledge of amino acids based primarily on their structures.
As well as giving me a chance to display my artistic skills, this quiz tests your knowledge of amino acids based primarily on their structures. 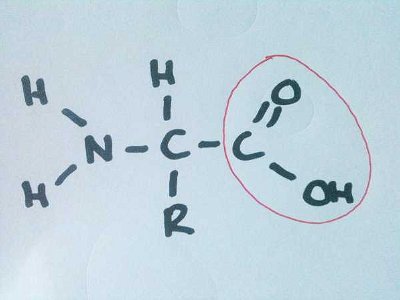 Never mind the complex names of all those proteins. Let's focus on what makes a protein - the amino acid. How much do you know about this building block of life?
Never mind the complex names of all those proteins. Let's focus on what makes a protein - the amino acid. How much do you know about this building block of life? 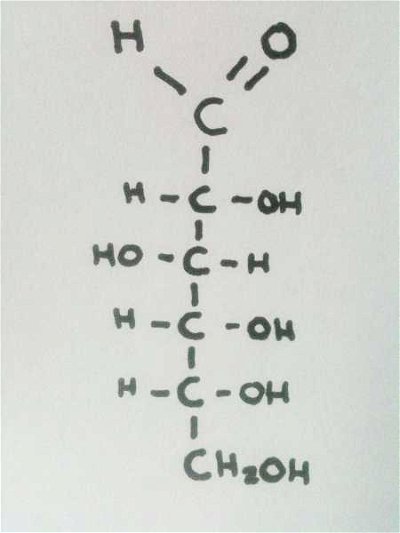 This quiz aims to provide a basic but challenging overview of the structure of simple sugars.
This quiz aims to provide a basic but challenging overview of the structure of simple sugars.  Quick Question
Quick Question = Top 5% Rated Quiz,
= Top 5% Rated Quiz,
 Top 10% Rated Quiz,
Top 10% Rated Quiz,
 Top 20% Rated Quiz,
Top 20% Rated Quiz,
 A Well Rated Quiz
A Well Rated Quiz