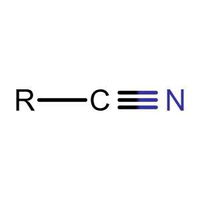
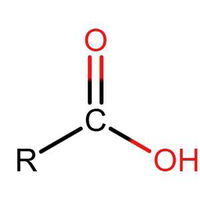
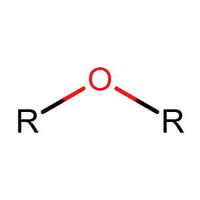
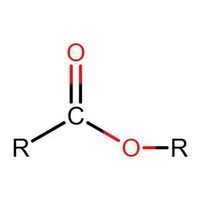
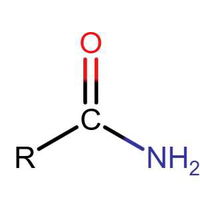
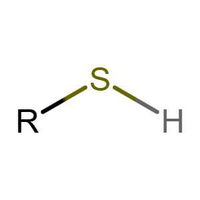
Identifying Functional Groups In Organic Chemistry Quiz
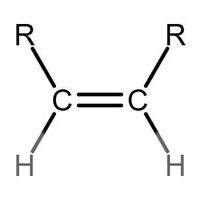
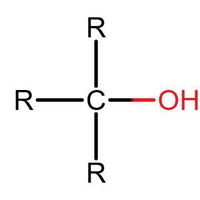
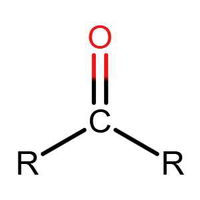
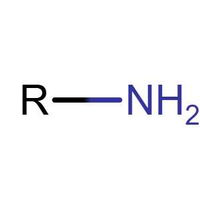
Many people can make little or no sense of diagrams of organic molecules. But even the most complicated molecules feature recognisable "functional groups", configurations of atoms that have specific chemical properties. See how many you can recognise.
by reeshy.
Estimated time: 3 mins.